|
All SKCCC CTO information has been moved to the SKCCC CTO ONE Site Oncology Study Start-Up
Process |
|||||||||||||||||||||||||||||||||||
|
The information provided covers the fundamental elements of initiating any oncology-related study at the Sidney Kimmel Cancer Center, from concept to activation. This will include the required committees and the sequence of committee reviews, key events that need to occur, and helpful checklists that will assist in making sure that all necessary activities have been completed prior to activation. The purpose of the study start-up process is to make sure that all oncology-related studies are being properly presented and discussed at all the required committees, and that the process of activating these studies is being done in an efficient and timely manner. Is my study a CANCER TRIAL? |
|||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||
|
Committee Approvals Depending on the type of study that is being initiated, will depend on which committees are required to review the study prior to submission to the Institutional Review Board. Each committee has a specific scope in which they review and approve studies, and approval from another committee may be required before the next committee can review and approve that study to move forward. The diagram below illustrates the four main committees that may need to review the study, and the committee sequence that is required. Note that retrospective chart reviews and correlative lab trials that will utilize banked blood and tissue that do not require informed consent do not require review or approval from a Multidisciplinary Disease Group or the Protocol Facilitation Committee. For details on how to submit to these committees, please visit the respective Web page. |
|||||||||||||||||||||||||||||||||||
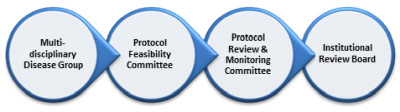
Click on bubbles above for more info. After Committee Approvals Site Initiation Visit (SIV) The Site Initiation Visit provides protocol training for all personnel involved in the study. For Investigator Initiated and NCI National Clinical Trials Network (NCTN) sponsored trials, the SIV is conducted internally, and for industry-sponsored trials, it is conducted by the sponsor. This visit should occur after the Institutional Review Board has approved of the study, budget and contract (if applicable) are agreed upon, and any other outstanding requirements have been resolved. Activation Activation of the trial occurs once an SIV has occurred, all applicable items on the start-up checklists have been completed, and any outstanding issues and concerns from the Protocol Facilitation Committee have been addressed. See Activation Procedures, Activation Announcement Email, and Clinical Trial Announcement for process. |
Note: In order to reduce time to activation, it is highly recommended that all Institutional Review Board submission paperwork be prepared prior to PRC submission.
|
||||||||||||||||||||||||||||||||||
Helpful Tools The following templates and checklists have been created to assist in activating studies in a timely and efficient manner. It is highly recommended that these templates and checklists be used throughout the study start up process where applicable. This document was created to assist investigators in writing investigator-initiated trials. The template includes all the required SKCC language as well as an outline and guidance as to what information is needed to be included in the protocol. Study Start-Up Checklist - Clinical This checklist was created to help identify all the required clinical coordination aspects of the study start process, as well help determine when a trial is ready to be activated. Study Start-Up Checklist - Regulatory This checklist was created to help identify all the required
regulatory coordination/essential documents of the study start process, as
well as help determine when a trial is ready to be activated. Note that there
is a second Study Start-Up Checklist – Regulatory, for multi-site studies
where SKCC is the lead site. |
|||||||||||||||||||||||||||||||||||

